Last updated on September 4th, 2024.
I will be the first to admit that it took me quite some time to truly understand what is heavy cream?
But whether we pour it into our coffee, cream our pasta sauces with, crown our ice cream with it, fill our Brioche donuts with, or frost our black forest cupcakes with, we all know and love the taste, smell, and texture of smooth and rich cream.
Learn How long does heavy cream last ? including some tips and recommendations for handling and longer shelf life.

I am super excited to share with you this post and take you on a short yet, informative journey into the wonderful world of baking science. If you are hungry for more, check out my book (release march ’22) “Baking Science” where I explore all aspects of it.
What is Heavy cream?
If you ever wondered how heavy cream is made, know that it’s by reducing the fat from fresh milk. So heavy cream is basically what we get when we separate milk and fat.
To reduce milk’s fat percentage, the milk is left still and uninterrupted. The absence of movement allows the fat globules to make their way to the surface (fat is a substance that does not like to be in the water, just like a salad dressing that has been sitting for a while and we can notice the top layer of fat). The thick fatty layer that accumulates at the top is the cream.
Heavy cream vs heavy whipping cream
The second most asked question right after “What is heavy cream?” is without a doubt “is heavy cream the same as heavy whipping cream?”.
The main difference between heavy cream and heavy whipping cream is the fat content. When the fat percentage is between 20-30% we tend to refer to it as cream, light cream, or fresh cream. Between 30-35% it is light whipping cream. 35% fat is known as whipping cream, and 36-38% is heavy whipping cream (Also known in Great Britain as Double cream).
Fortunanetty, nowadays all types of cream are easily found in our local grocery store, in the dairy aisle.
Whipping cream
A cream will get its title “whipping cream” if the fat percentage is greater than 30%. It means that the cream is rich in fat globules that can trap tiny air bubbles and essentially create a thick and stable foam.

What is Heavy Whipping Cream?
Heavy whipping cream is cream with a fat content of 36-38%.
The “fattier” the cream is the richer, thicker, with stiff peaks the whipped cream will be. Learn how to stabilize whipped cream, using 5 different methods.
Pasteurised vs. Ultra pasteurized cream
To pasteurize means to extend the shelf life of milk and cream by applying enough heat that will kill and inactivate living cells and enzymes that could cause food poisoning.
Pasteurised
Heating to 145F/62C for 30-35 minutes OR 162F/72C for 15 minutes.
Ultra pasteurised
Heating the to 265-300F/130-150C for 1-3 seconds.
The difference in time and temperature also means a difference in flavor and a change in the fat’s molecular structure.
The higher the temperature the more “cooked” the flavor is. In addition, the high temperature of ultra-pasteurized products also causes the fat molecules to tear apart into smaller fat globules. While it is great for smooth and cohesive emulsification for our milk with no fat lumps at the top, it is not ideal for heavy whipping cream.
Ultra-pasteurized cream has a thin texture similar to half-and-half, and the smaller fat globules lose some of their ability to trap air and stabilize the whipped cream.
So ultra-pasteurized cream is great for cooking but not the best option for whipping.
The process of whipping cream, and the science behind it
My guess is that you are here because you are a curious baker who wants to improve your baking skills. I found that the best way to become a better baker is by understanding the makeup of our ingredients and taking a closer look at the molecular level. This is exactly what I did here, and for better understanding, I even added a few illustrations. So let’s get started.
If we take a picture of the different molecules in 1 cup of Heavy whipping cream before whipping it, we will see some fat globules, swimming randomly in the water. Even though fat and water don’t mix, those fat globules do not float to the top because they are attached by the protein which emulsifies (bonds) the fat in the water. (see illustration 1.1)
This is the process of emulsification that I talk about in my book “Baking science”.
By whipping the cream we essentially add air. As we introduce air into the heavy cream, the fat globules surround the air and trap it in the shape of air bubbles (see illustration 1.2). The more we whip the more air we incorporate and the smaller the bubbles get. And the more air bubbles we incorporate the less space there is for the water to move. So we end up with a light foam, rich in air, fat, and proteins. (see illustration 1.3)
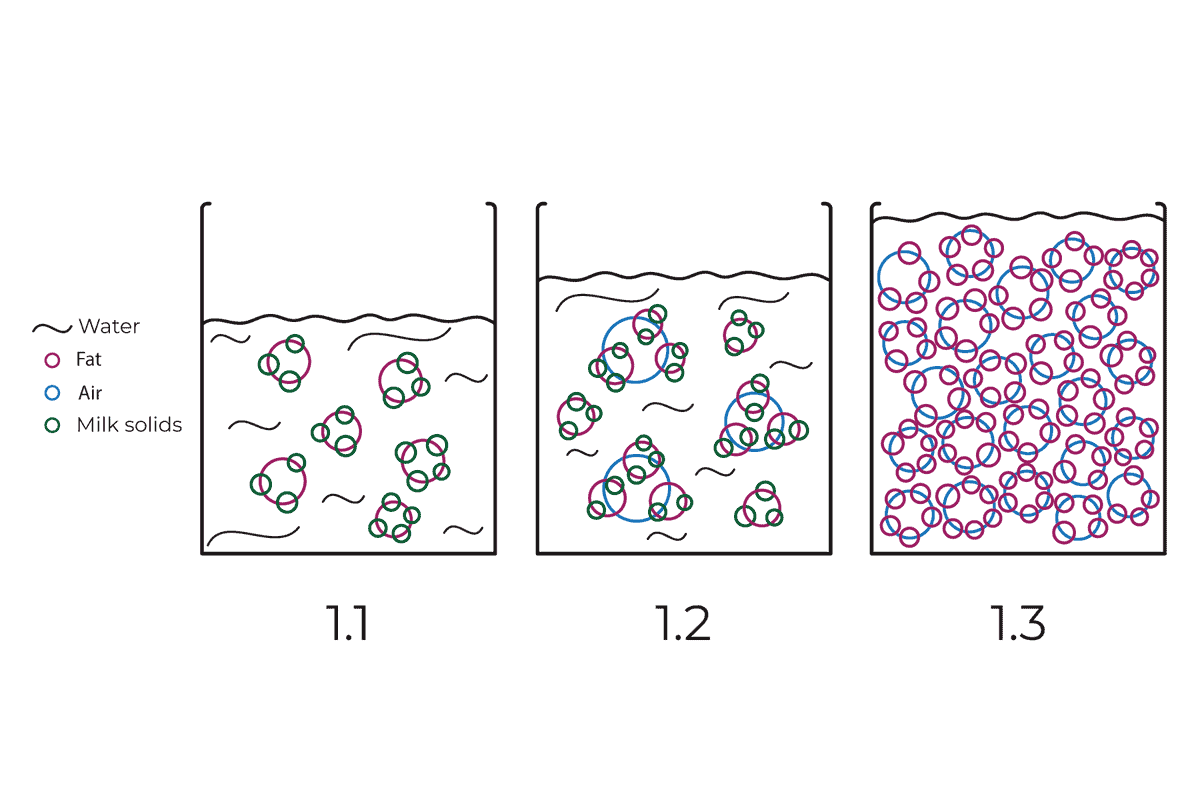
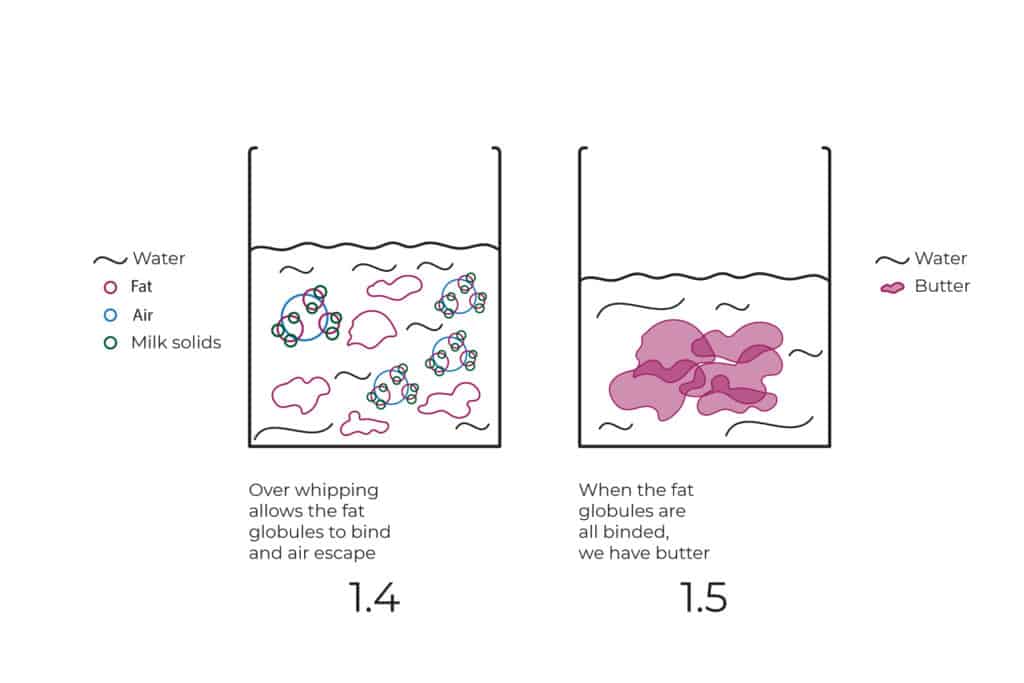
The problem is when we keep whipping even after we have reached the max capacity of air. When that happens, then the overcrowded environment pushes the fat globules closer together and forces them to bind. As a result, they let go of their hold of the bubbles, allowing the air to escape and the cream to deflate. (see illustration 1.4)
The more we whip the closer the fat globules will get and the more they will bind together. So by the end of the day, our heavy cream will turn into butter. (see illustration 1.5)
The science of stabilized heavy cream
Although many bakers have mastered the art of whipping heavy cream, they still find it a source of frustration.
Many times the cream is just not stable enough to fill, frost our cakes, or even pipe it on our pies or cupcakes and as soon as we apply it to our baked goods it turns flat and many times even melts and drips.
This is why we add some type of stabilizer to firm and strength the texture of our whipped cream.
Type of stabilizers:
Even though there are quite a few substances we can use as stabilizers, you might be surprised to know that there are only two ways of stabilizing whipped cream:
- Creating a crowded environment. The gaps between the air bubbles and fat globules are filled with water. Water is the heaviest substance in the cream which is why it can easily weigh our air down. By filling the gaps with a different substance we eliminate that risk. Stabilizers like Cornstarch, Xanthan Gum, Gelatin, and cream of tartar are great examples.
- Strengthening the emulsification. Illustration 1.4 has demonstrated how easily the fat globules bind together causing the cream to deflate. What if we will add a layer of protection around the fat globules that will make it hard for them to bind? This is exactly what we do when we add Dry milk powder.
How do the stabilizers work?
We are now getting really into the nitty and gritty and diving deep into the process of how each substance stabilizes the cream. We do that by taking a close look at the molecular level.
Dry Milk Powder
I decided to start with this one, well, because this is my favorite method of stabilizing heavy cream. (I actually used it to make Caramelized Whipped Cream in my book)
To stabilize heavy cream, we add up to 1 ½ tablespoon of non-fat dry milk powder to every 1 cup of heavy whipping cream.
Why?
Dry milk powder is the milk solids we get when we remove the water content from milk. The solids are made of sugars and different types of proteins.
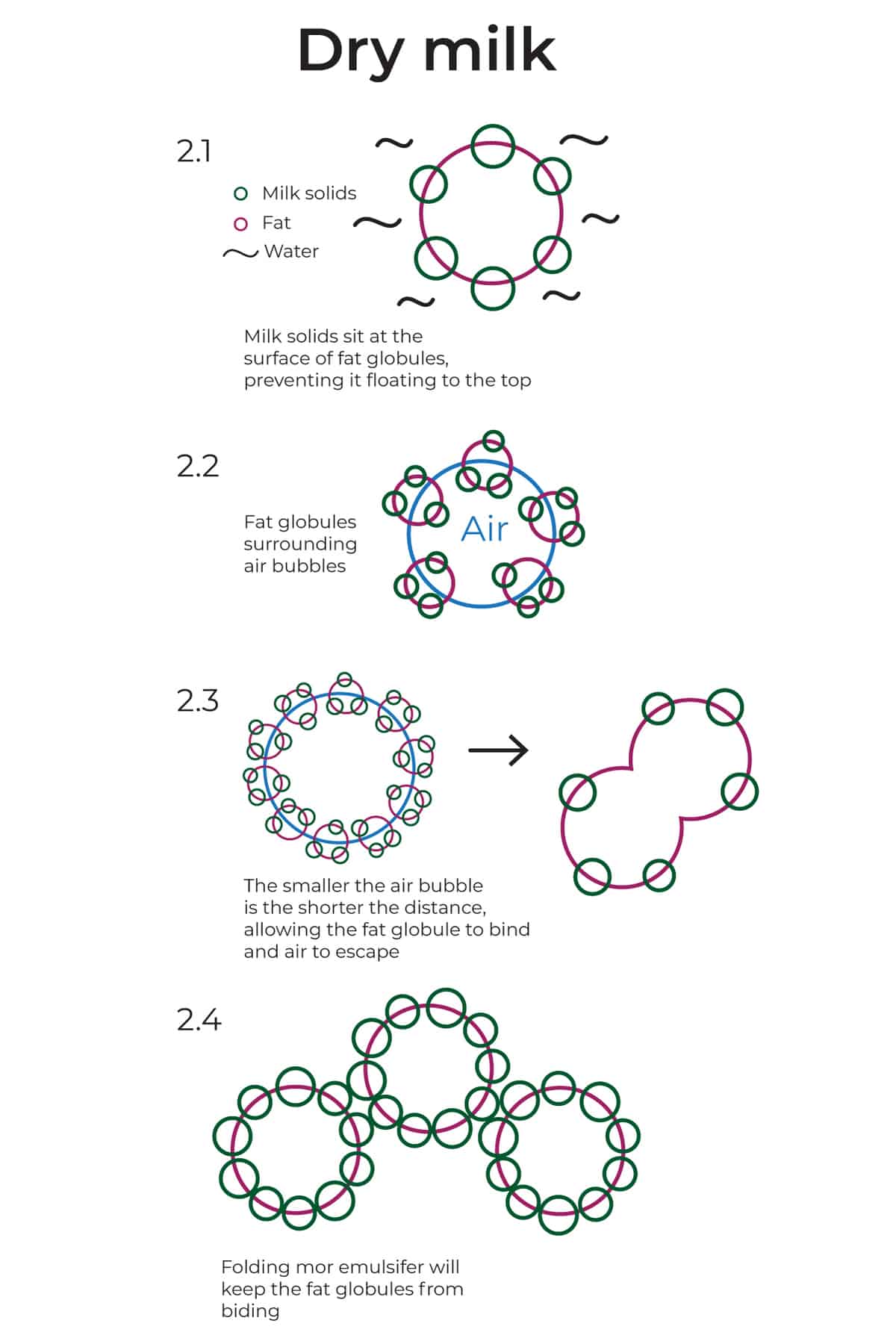
What is incredible to know about dry milk powder is that it also functions as an emulsifier, and helps the fat in our cream to stay in the water and not float to the top.
It does that by sitting at the surface of the fat globules, preventing them from binding with other globules, or/and finding their way to the top. (See illustration 2.1)
When we incorporate air, the fat globules sit at the surface of the air in the shape of bubbles. (see illustration 2.2).
As we whip, the air bubbles get smaller and smaller, and so is the distance between the fat globules. At some point, the fat globules get so close to each other they bind. As they do, the air escapes and the cream deflates. (See illustration 2.3)
By adding dry milk powder we increase the number of emulsifiers that sit around the surface of the fat globules, creating a sort of “protection wall”. The more emulsifiers we have the less likely the fat globules will have a chance to bind. (see illustration 2.4)
Cornstarch
Using starches and especially cornstarch as a way to thicken and stabilize our baked goods, is very common.
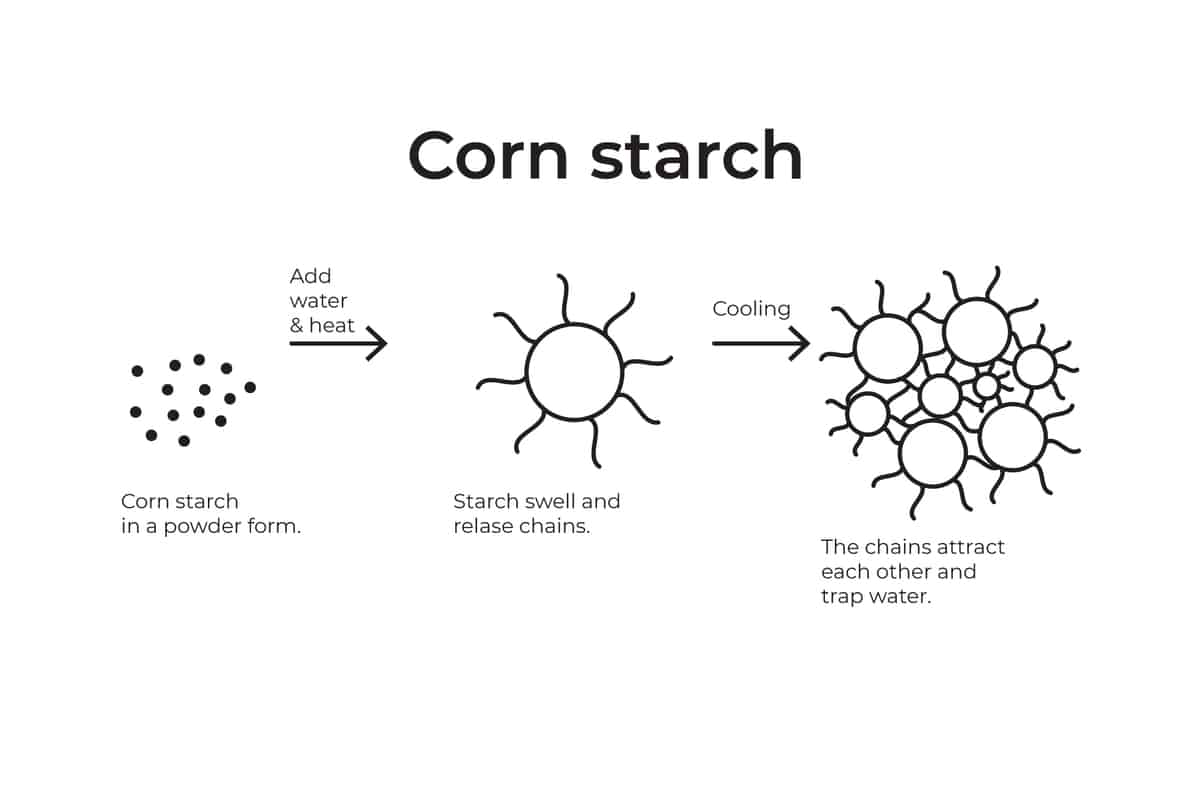
Starches granulate are like tiny sponges that soak up water in the presence of heat.
As they soak up the liquids two main things occur:
- They increase their volume and reduce the “free space”.
- They release long carbohydrates chains that attract each other and bond. As they bond they trap water between the gaps (this process is also known as “Gelation”)
Once both elements are present, the fluidity of the water in the heavy cream has decreased leaving us with a thick and stable whipped heavy cream.
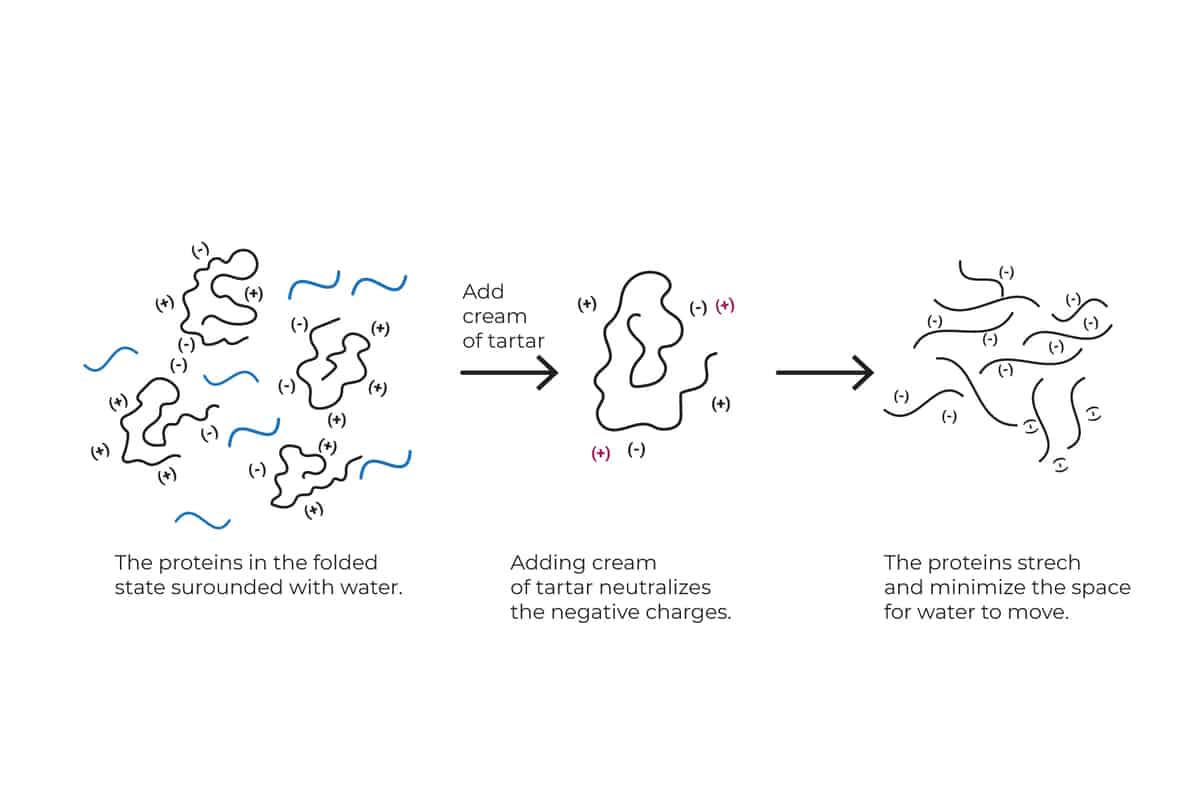
Cream of tartar
You might already know this process from an experiment you may have done during your school years.
Most substances have a positive charge (+), a negative charge (-), or both.
We all learned in school that a positive charge (+) attracts a negative charge (-), when that happens the charges are neutralized. On the other hand, negative charges repulse negative changes and positive charges repulse positive charges. The best example is our home remote. We place the batteries in opposite directions so the positive charge will face the negative charge.
Milk proteins are long chains of amino acids made of some positive charges and some negative charges. In their natural state, they both repel and attract each other and maintain a safe distance from each other in a folded state. Because they are folded and away from each other, the water has plenty of room to move around, giving our heavy cream a thin texture (fluid).
Cream of tartar is an acidic substance with positive charges. So when we add cream of tartar to our heavy cream, the positive charges from the cream of tartar will neutralize the negative charges in the milk protein and leave it with nothing but positive charges which will repel each other. As they do, the chains will unfold and take up much more space and create a crowded environment, trapping the water in the gaps.
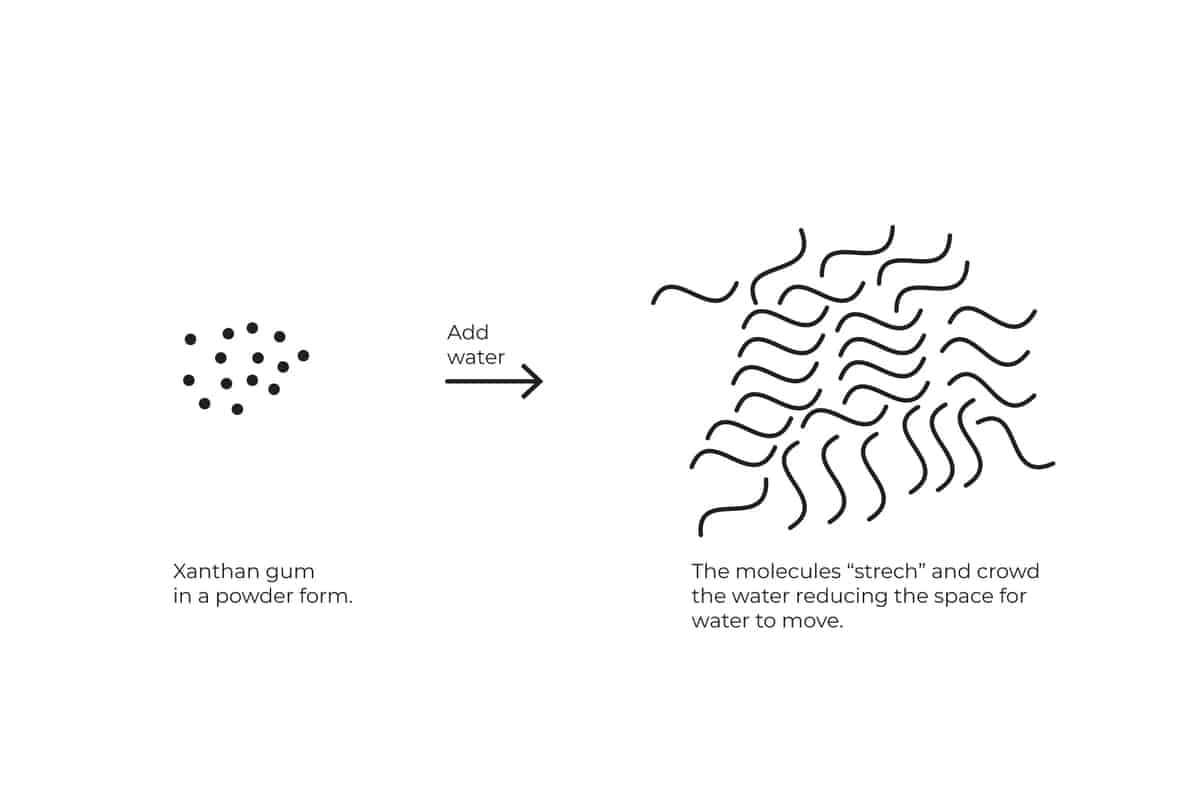
Xanthan Gum
Xanthan Gum is a very concentrated powder of tiny tiny molecules. In their dry state, the molecules are folded and close to each other. When mixed with liquid they “stretch” and break apart.
As they do they crowd the environment and reduce the fluidity.
Note: since Xanthan gum is so concentrated the recommended amount is up to 0.01% of the liquid weight.
One cup of heavy cream is about 80-100 grams of liquid, so we should use up to and no more than 1 gram.
A ¼ tsp is 0.7 grams and is the recommended amount.
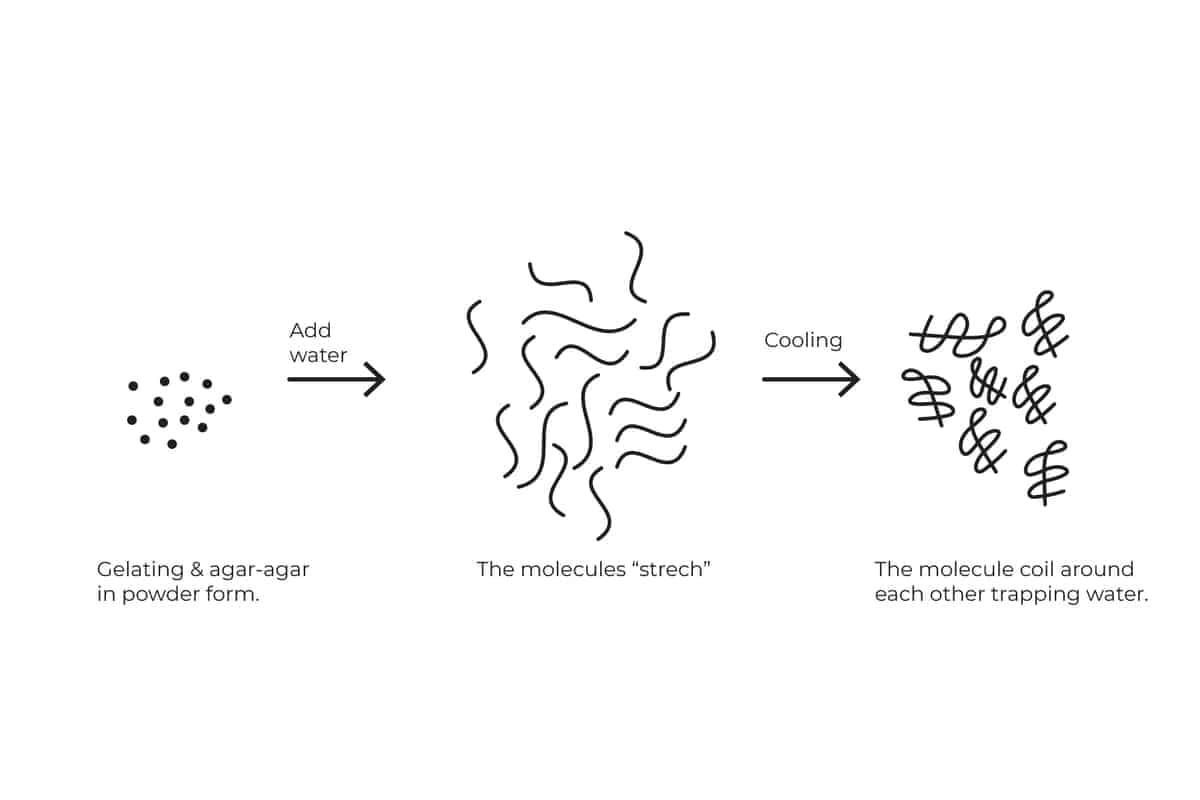
Gelatin and Agar-Agar
Gelating (animal source) and Agar-Agar (plant source) are a combination of all of the above. Both gelatin and Agar-Agar are powders that in the presence of liquid, their molecules “wake up and stretch” as they do they occupy a lot of the free space.
In addition, as they cool, they coil around each other and trap even more liquid in between the gaps.
Conclusions and expert tips
Be sure to carefully read the below point for extra tips and helpful information.
- Called by many names. Heavy cream has many different names and each name is an indication of the fat content in the cream.
- It’s an emulstion. Heavy cream in an emulsion of fat with liquid. All emulsions eventually break, so when you notice a thick layer of fat in the heavy cream container shake it vigorously to re-emulsify.
- In the United States, cream with a higher fat content is referred to as Heavy whipping cream and it has the highest fat contents.
- In the UK cream with a high fat content is known as Double ream.
- Fat melts easily in a warm environment which is why it is super important to refrigerate the cream. For best results when whipping cream, cooling the metal bowl and whisk will help stabilize the cream.
- Make your own. Butter is made when the fat is separated from the cream. Therefore, if you would like to make your very own heavy cream substitute, mix in 1 stick melted butter with ½ cup of whole milk or half and half and 1 tsp of dry milk powder. Allow cooling before whipping.
- Heavy cream is a common ingredient used in many of your favorite recipes such as pastry fillings, crème fraîche (heavy cream mixed with sour cream), creamy soups, and even panna cotta. However, it is a dairy product with an expiration date.
- There are many different brands, the expensive brands do not employ better results. I found that the off brands are usually a better option and many times is the best option for a better deal.
- To make your very own half and half, simply mix equal parts of cream and milk (half cream with half milk).
Illustrations by Camila Rojas.
Here are some delicious recipes using heavy cream
Butter Cream Cake , Walnut Chocolate tart, Black Forest Cupcakes, Brioche Donuts.



I missed a discussion of pasteurized vs. ultra-pasteurized heavy cream and fat content.
You are 1000% right, I added a few words about that.
Thank you I think this article really covers all the aspects of cream. When making ice cream I prefer pasteurized heavy cream. Trader Joe’s carries it but many stores do not.
That is very interesting, I am only aware of 1 product at trader Joe’s and lately, they haven’t even had any…
A piece like this on butter and all that is available and how they differ when baking would be interesting.
Yes! absolutely in the future, right now I am working on Tempering chocolate.
Thank you very much for a clear thorough explanation.
Will your book be published in Hebrew too?
Thank you Michal glad you enjoyed it, for now, my book is only in English, maybe one day!